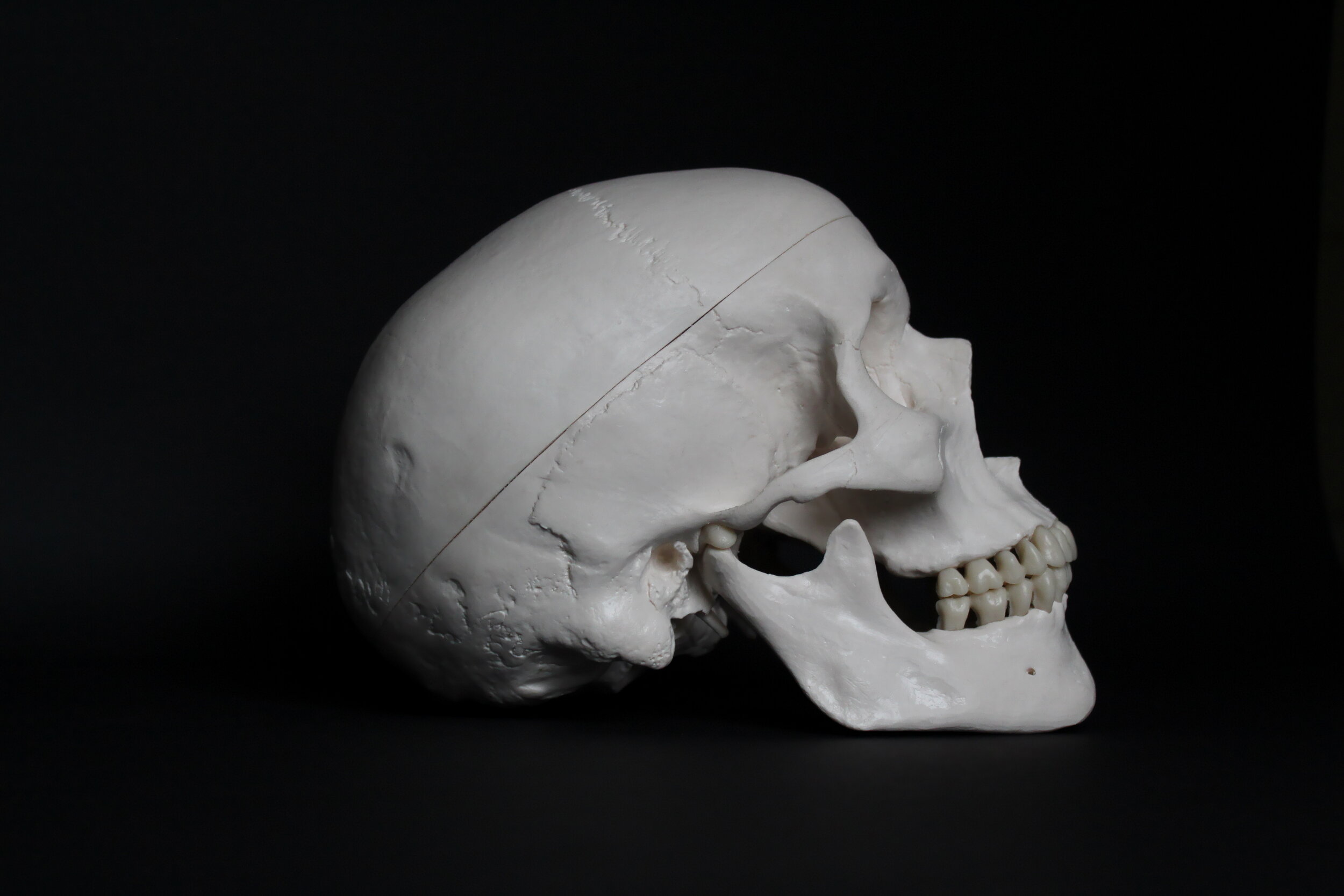
Cranial Anatomy
The skull is made up of 22 bones: the cranium includes eight bones that surround and protect the brain and 14 bones that form the face. In adults, all but one of the skull bones are fused together by immovable joints called sutures. The sutures lock the edges of the skull bones together, like pieces in a puzzle, to form a structure that is both rigid and strong. The mandible, or lower jaw, the only moveable skull bone, allows the mouth to open and close. In newborns, the skull bones are not completely fused; they are linked by fibrous membrane called fontanels. Fontanels allow the skull to be compressed slightly during birth and accommodate growth of the brain during early infancy. By one-and-a-half years of age, the skull sutures have formed and the fontanels have disappeared.
Cranial Bones
The frontal bone forms the forehead. Two parietal bones form the sides of the cranial roof. Two temporal bones form the lower cranial sides. The occipital bone forms the cranial rear and floor. The ethmoid bone forms part of the nasal cavity. Shaped like a butterfly, the sphenoid bone forms the middle part of the cranial floor.
Facial Bones
The 14 facial bones provide the structure of the face and form the openings through which food, water, and air enter the body. Each of the following facial bones are paired: the maxillae form the upper jaw and front of the hard palate; the zygomatic bones form the cheeks; the nasal bones form the bridge of the nose; the lacrimal bones form part of the orbit, or eye socket; the palatine bones form the rear of the hard palate; and the inferior nasal conchae divide the nasal cavity. The vomer is a single bone that makes up part of the nasal septum, which divides the nostrils, and the mandible forms the lower jaw. The maxillae and mandible secure the teeth. Small holes in the skull bones, called foraminae, and canals enable blood vessels, such as the carotid arteries and nerves, to enter and leave the skull. The spinal cord passes through a largest hole, called the foramen magnum, in the base of the cranium to join the brain. The occipital condyles on either side of the foramen magnum articulate with the first vertebra (C1) of the spine to permit up-and-down movement of the head.
The Brain
The brain is the control center of the nervous system. It enables us to think, feel, and move. The brain constantly receives information and sends out instructions to the body through the spinal cord and the body's vast network of nerves.
There are 12 pairs of cranial nerves branching off the brain. These nerves relay impulses from the sensory organs, such as the eyes or ears. Thirty-one pairs of spinal nerves branch off the spinal cord, exiting between each level of vertebrae. These nerves relay impulses to and from the rest of the body.
The largest part of the brain is the cerebrum, which controls the most sophisticated functions, such as thought, imagination, memory, emotion, speech, and sensory perception. The human cerebrum is quite large. It has two halves, or hemispheres. A band of nerve tissue, called the corpus callosum, links two halves to allow them to exchange information. Each hemisphere is covered by a layer of gray tissue, called the cerebral cortex, which is responsible for the higher functions of the brain, including conscious thought. The cortex is composed of sulci (folds) and gyri (bulges), which together provide a large surface area in the limited space inside the skull.
The cortex of each hemisphere has four lobes: the occipital, temporal, parietal, and frontal lobes. The occipital lobe controls vision. The temporal lobe controls sound and speech. The parietal lobe controls movement, touch, and recognition. And the frontal lobe controls thinking and planning.
The brain stem and hypothalamus control automatic processes, such as breathing and heartbeat.
The cerebellum acts as a "mini brain" that coordinates body balance, posture, and movement.
The human brain is well protected from injury. It is firmly surrounded by three layers of membranes, encased in a rigid skull (the cranium), and covered by a muscular scalp. Each of these barriers to the brain is important, because brain tissue is fragile and unforgiving if injured.
Three membrane layers, the meninges, protect the brain from injury and infection. The dura mater, tough and fibrous, lines the skull. The thinner pia mater, highly vascular (containing many blood vessels), covers the brain's surface. Between these two is another, the arachnoid. The brain floats in a protective cushion of cerebrospinal fluid (CSF), which flows within the subarachnoid space-beneath the arachnoid membrane, on top of the pia mater. It also surrounds the spinal cord and fills open spaces (ventricles) inside the brain. The amount of CSF that circulates around the brain normally stays the same, replenished by the body, and helps to maintain a constant pressure inside the skull, known as intracranial pressure (ICP). The largest part of the brain is divided into two major areas, the left and right cerebral hemispheres, which control most of the body's thought and sensory processes. Some sections of each hemisphere can be "mapped" to correspond with the body functions for which they seem to be responsible. The brain stem controls such vital functions as breathing, heartbeat, and eye movement. It anchors the brain to the other part of the central nervous system, the spinal cord, and acts as the main circuit for all brain activity. Twelve pairs of cranial nerves, emerging from the base of the brain and the brain stem, transmit nerve impulses for vision, hearing, smell, and many other important body functions.
The Skull
A tumour is an abnormal mass of tissue that grows on or inside the body. It is known as primary if located where its growth first started, or secondary if it began growing elsewhere in the body and metastasized, or spread, to its present location. Most primary brain tumours do not metastasize outside the brain. Inside the skull, tumours can grow almost anywhere: within brain tissue, from the meninges, or inside the ventricular system. They can be encapsulated (self-contained) or interwoven with blood vessels, nerves, or other brain structures from which they cannot be removed without devastating consequences. Metastatic tumours are usually well localised, may occur alone or in clusters, and may spread throughout much of the brain. A benign tumour usually is encapsulated, does not spread to other areas of the body, grows slowly, and often causes problems by compressing brain tissue. A malignant tumour grows uncontrollably, spreads throughout the brain, and destroys brain tissue.
What symptoms can it cause?
A brain tumour may at first cause the vague feeling of being "unwell." This may be followed by other, more specific symptoms: dull, persistent headache; nausea or vomiting; generalized weakness; vision problems. Because the left side of the brain governs the right side of the body, and vice versa, a tumour will cause specific weakness or loss of movement on the opposite side of the body. Some symptoms may be caused by the increased pressure inside the skull from brain swelling, which can temporarily be treated with a steroid medication. Because brain tissue is irritated by the tumour, the brain can temporarily "short-circuit" as its normal electrical activity is interrupted. These periods of uncontrolled brain activity can cause seizures, which may be generalised and cause contractions of all parts of the body, loss of consciousness or bladder and bowel function.The seizures may instead be of a focal nature, affecting only one arm, a leg, or part of the face. Seizures usually can be controlled with anticonvulsant medications.
How is it evaluated?
A detailed history-taking of the patient's symptoms and a physical examination are done first, followed by any of several tests, such as x-ray studies, Computerised Tomography (CT) scans, Magnetic Resonance Imaging (MRI), and angiograms. All findings are used to evaluate the patient's symptoms, determine the tumour's exact location, and provide the physician with a tentative diagnosis of the tumour type. During surgery, ultrasound imaging may be used to pinpoint the tumour's precise location and help the surgeon plan his approach for its removal. If an emergency craniotomy is required, an extensive workup may not be possible.
Brain Tumours
Meningioma
Growing from abnormal cells of the meninges, meningioma is a slow-growing tumour that shares the dura's rich blood supply. It is very often attached to dura and so may be immediately visible when the dura is opened.
It is usually a benign tumor and well encapsulated, but removal may be complicated by its size, firmness, and attachment to vital blood vessels or brain tissues. A large meningioma or one that is difficult to remove may require a long, tedious surgery and can cause further brain swelling and blood loss. Often the dura removed during tumour surgery may be replaced with other body tissue (fascia) or a dura substitute.
Glioma
Glial cells support the brain's functioning nerve network and are the site of tumours inside the brain. Gliomas are "graded" according to their degree of malignancy. Often when the dura is opened, the brain is swollen but otherwise may appear normal. The "centre" of the glioma may readily be identified, but because the tumour gradually spreads into surrounding tissue the boundaries of a glioma are harder to identify.
It may take months for the cells around the edges of the tumour to appear abnormal, yet they can be affected long before they "show" themselves. This is why glioma usually cannot be removed completely, as even one remaining cell can continue the tumour's growth.
Metastatic Tumour
Often lying close to the brain's surface, where it irritates the normal tissue around it, a metastatic tumour is one that began in another body organ and traveled in the bloodstream to the brain. Grown from a "seed" of non-brain tissue (from the breast, kidney, or lung, for example) a metastatic tumour often can be separated from the surrounding brain more easily. If only a single lesion exists, all or part of it usually can be surgically removed.
Haemorrhage is the medical term for bleeding. The rupture of one of the brain's blood vessels can cause bleeding into the subarachnoid space - beneath the arachnoid membrane, on top of the pia mater - and into brain tissue. The bleeding usually stops, at least temporarily, when a clot forms over the ruptured area.
Why does it happen?
The most frequent cause of spontaneous subarachnoid haemorrhage (not due to injury) is the rupture of a small aneurysm, or bulging sac, on one of the blood vessels that supplies the brain. It is usually impossible to determine why the aneurysm forms and bursts, but the condition is common in adults and may be associated with aging, diabetes, pregnancy, hypertension (high blood pressure), heredity, or trauma.
Cerebral aneurysms are usually of three types: saccular with a narrow "neck" (called "berry" aneurysms because of their shape and their tendency to occur in clusters); saccular with a broad base; and fusiform, in which a short section of the artery bulges all the way around. Each shape determines the degree of difficulty a surgeon faces in attempting to treat the problem.
An aneurysm ruptures spontaneously - even during sleep - and therefore is not related to the strain of hard work, sexual intercourse, or other physical activity.
Although it is not always possible to discover the exact source of bleeding, other causes of spontaneous subarachnoid haemorrhage include: arteriovenous malformations, small angiomas, certain types of infections, and bleeding disorders.
What symptoms can it cause?
A ruptured cerebral aneurysm at first causes severe headache, which can be followed by nausea, vomiting, double vision or sensitivity to light, neck pain or stiffness, weakness, memory loss, paralysis, coma, or death. How severe the symptoms are and how long they last will depend on the amount and location of the bleeding. Blood in and around the brain can cause pressure, swelling, and brain irritation, which can lead to drowsiness, confusion, weakness or paralysis, memory loss, speech problems, behavior changes, or coma (complete loss of consciousness).
What complications can occur?
The blood vessels around the aneurysm are irritated by the blood from the haemorrhage and will at times go into a state of spasm, tightening and narrowing. This vasospasm ("vaso" meaning vessel) can occur any time after the rupture until the haemorrhaged blood has been absorbed by the body, and it can increase any or all symptoms. It is the body's own attempt to prevent a second haemorrhage by restricting the flow of blood through the vessels around the aneurysm. Vasospasm thus reduces pressure on the delicate aneurysm but unfortunately also reduces the normal blood supply to parts of the brain.
Ongoing research is being done to discover a medicine that will control vasospasm; as yet, none has proven effective. Other complications from subarachnoid haemorrhage, such as hydrocephalus, haematoma (blood clot), and brain swelling, involve the brain; but other body systems can also be affected because of the severe nature of the illness. Pulmonary embolus, heart abnormalities, and bleeding from an ulcer may cause further complications.
How is it diagnosed?
Several tests are used to confirm the diagnosis of ruptured cerebral aneurysm. Some are explained in the other parts of this web site.
Because cerebrospinal fluid flows within the subarachnoid space, a sample of CSF taken during a spinal tap at the base of the spine will show blood from the haemorrhage.
A CT scan will show blood inside the skull and indicate how much bleeding has occurred.
To find the source of the haemorrhage, an angiogram is performed, which may have to be repeated to try to pinpoint the aneurysm's exact location.
Hospitalisation
ActivityBecause the aneurysm can rupture again, a quiet, restful atmosphere is important. The patient usually is placed in the Intensive Care Unit (ICU), a highly specialised area providing close observation with specialised nursing care. Complete bed rest without physical strain is essential while the patient's condition stabilises - usually in preparation for surgery.
MedicationsMedications will be given when necessary to reduce pain, control blood pressure, relieve stress, and maintain fluid balance.
Breathing
If necessary, a respirator may be used to help the patient breathe and to control intracranial pressure (ICP). Most often, however, oxygen is merely administered to the patient through nasal prongs or a mask.
Monitoring devices
Various monitoring devices may be used to assess the patient's condition during recuperation. Among the most common are: an ECG (heart) monitor, an ICP (Intracranial Pressure) monitoring device, a Swan-Ganz catheter to assess the patient's fluid balance, and an arterial line to continuously measure blood pressure and aid in drawing frequent blood samples for laboratory study.
Nutrition
Intravenous (I.V.) fluids may be given until liquids and food can be taken adequately by mouth. The amount of fluid given will be closely monitored until the dangers of brain swelling (edema) and vasospasm lessen.
Subarachnoid Haemorrhage
Hydrocephalus is a condition in which excess cerebrospinal fluid (CSF) builds up within the ventricles (fluid-containing cavities) of the brain and may increase pressure within the head. Although hydrocephalus is often described as "water on the brain," the "water" is actually CSF, a clear fluid surrounding the brain and spinal cord. Cerebrospinal fluid (CSF) is formed in a region of the brain known as the choroid plexus. CSF has three crucial functions:
1) it acts as a "shock absorber" for the brain and spinal cord;
2) it acts as a vehicle for delivering nutrients to the brain and removing waste;
3) it flows between the cranium and spine to regulate changes in pressure within the brain.
The average adult produces about half a litre of CSF daily. When an injury or illness alters the circulation of CSF, one or more of the ventricles becomes enlarged as CSF accumulates. In an adult, the skull is rigid and cannot expand, so the pressure in the brain may increase profoundly. Hydrocephalus is a chronic condition. It can be controlled, but usually not cured. With appropriate early treatment, however, many people with hydrocephalus lead normal lives with few limitations. Hydrocephalus can occur at any age, but is most common in infants and adults age 60 and older. It affects adult males and females, as well as people of different races about equally. Experts believe that normal pressure hydrocephalus accounts for 5 to 6 percent of all cases of dementia.
The incidence is approximately 1 out of 1,000 people. Hydrocephalus most often occurs in children, but may also occur in adults and the elderly.
Hydrocephalus Ex-Vacuo
Hydrocephalus ex-vacuo occurs when a stroke or injury damages the brain and brain matter actually shrinks. The brain may shrink in older patients or those with Alzheimer's disease, and CSF volume increases to fill the extra space. In these instances, the ventricles are enlarged, but the pressure is usually normal.
Normal Pressure Hydrocephalus (NPH)
NPH results from the gradual blockage of the CSF draining pathways in the brain. The ventricles enlarge to handle the increased volume of CSF, and the compression of the brain from within by the fluid-filled ventricles destroys or damages brain tissue. NPH owes its name to the fact that the ventricles inside the brain become enlarged with little or no increase in pressure. However, the name can be misleading, as some patient's CSF pressure does fluctuate from high to normal to low when monitored. NPH can occur as the result of head injury, cranial surgery, haemorrhage, meningitis or tumour. Unfortunately, the cause of the majority of NPH cases is unknown, making it difficult to diagnose and understand. Compounding this difficulty is the fact that some of the symptoms of NPH are similar to the effects of the aging process, as well as diseases such as Alzheimer's and Parkinson's. The majority of the NPH population is older than 60, and many of these people believe their symptoms are just part of the aging process. Unfortunately, many cases go unrecognised, are never properly treated, or are misdiagnosed.
What symptoms can it cause?
Symptoms vary depending on the cause of the obstruction to CSF circulation, the age at which the problem develops, and the extent of damage to brain tissue caused by the swelling.
Symptoms of Adult-Onset Hydrocephalus:
Headaches
Nausea
Difficulty focusing the eyes
Unsteady walk or gait
Leg weakness
Sudden falls
Irritability
Drowsiness
Personality changes
Seizures
Primary Symptoms of NPH:
Gait disturbance (difficulty walking)
Dementia or forgetfulness
Bladder control problems (as the condition progresses)
The gait in many patients with NPH is very distinctive: wide-based, short, slow and shuffling. People may have trouble picking up their feet, as if their feet are glued to the ground. They may have difficulty going up and down stairs and curbs, and as a result, they are frequently falling. Gait disturbance is often the most obvious first symptom. These disturbances range in severity, from mild imbalance to the inability to stand or walk at all. The symptoms of NPH usually get worse over time if the condition is left untreated. Patients with untreated, advanced NPH may experience seizures, which can get progressively worse. Dementia and/or bladder control problems usually appear after gait disturbances, as the condition progresses. Mild dementia can be described as a loss of interest in daily activities, forgetfulness, difficulty dealing with routine tasks and short-term memory loss. Not everyone with NPH develops an obvious mental impairment. Bladder control problems usually involve urinary frequency and urgency in mild cases. In severe cases, however, a complete loss of bladder control (urinary incontinence) may result. Urinary frequency is the need to urinate more than usual, often as frequently as every one to two hours. Urinary urgency is a strong, immediate physical need to urinate. This urge is sometimes so strong that it cannot be controlled, resulting in incontinence.
Diagnosing Hydrocephalus
Patients presenting the three primary NPH symptoms or a combination of the other symptoms should consult a neurosurgeon as soon as possible. Before your doctor can recommend a course of treatment, he or she will:
Review your medical history, and perform a physical examination
Perform a complete neurological examination including diagnostic testing if needed
Ask specific questions to determine if symptoms are caused by hydrocephalus
The neurological examination will also help to determine the severity of your condition. There are a wide variety of diagnostic tests that can help pinpoint the cause and severity of hydrocephalus.
Computed tomography scan (CT or CAT scan): A diagnostic image created after a computer reads x-rays; can show if the ventricles are enlarged or if there is an obvious blockage.
Magnetic resonance imaging (MRI): A diagnostic test that produces three-dimensional images of body structures using magnetic fields and computer technology; can reveal if the ventricles are enlarged and evaluate the CSF flow. The MRI provides more information than the CT scan, so is the preferred test, in most cases.
Isotopic cisternography: A test that involves injecting a radioactive isotope into the lower back through a spinal tap. This allows the absorption of CSF to be monitored over a period of time (up to 4 days). Isotopic cisternography is considerably more involved than a CT scan or MRI, but can aid in the diagnosis of NPH.
Lumbar puncture (spinal tap): Under local anesthetic, a thin needle is passed into the spinal fluid space of the low back. Removal of up to 50 cc of CSF is done to see if symptoms are temporarily relieved. This test is used to measure CSF pressure and analyze the fluid. This procedure may help determine whether a shunt, the common treatment for hydrocephalus, will work. If lumbar puncture improves symptoms even temporarily, this can be an indication that a shunt will be successful. There are patients, however, who show no improvement that go on to have a successful shunt procedure.
Intracranial pressure monitoring: Monitoring may be able to detect an abnormal pressure or pattern of pressure waves. Monitoring requires insertion of a catheter or small fiber optic cable through the skull into the brain. This procedure requires admission to the hospital for 24 hours.
When Surgery is Necessary
Hydrocephalus can be treated in a variety of ways. The problem area may be treated directly (by removing the cause of CSF obstruction), or indirectly (by diverting the fluid to somewhere else; typically to another body cavity). Indirect treatment is performed by implanting a device known as a shunt to divert the excess CSF away from the brain. The body cavity in which the CSF is diverted is usually the peritoneal cavity (the area surrounding the abdominal organs).
In some cases, two procedures are performed, one to divert the CSF, and another at a later stage to remove the cause of obstruction (e.g., a brain tumor). Once inserted, the shunt system usually remains in place for the duration of a patient's life (although additional operations to revise the shunt system are sometimes needed). The shunt system continuously performs its function of diverting the CSF away from the brain, thereby keeping the intracranial pressure within normal limits.
An alternative operation called endoscopic third ventriculostomy may be recommended. In this operation, a tiny burr hole is made in the skull and a neuroendoscope (a small camera which is attached to medical instrument) is utilised to enter the brain. The neurosurgeon will then make a small hole (several millimeters) in the floor of the third ventricle, creating a new pathway through which CSF can flow.
Recovery
Your neurological function will be evaluated post surgery. If any neurological problems persist, rehabilitation may be required to further your improvement. However, recovery may be limited by the extent of the damage already caused by the hydrocephalus and by your brain's ability to heal.
Because hydrocephalus is an ongoing condition, long-term follow-up by a doctor is required. Follow-up diagnostic tests including CT scans, MRIs and x-rays, are helpful in determining if the shunt is working properly. Do not hesitate to contact your physician if you experience any of the following postoperative symptoms:
Redness, tenderness, pain or swelling of the skin along the length of the tube or incision
Irritability or drowsiness
Nausea, vomiting, headache or double vision
Fever
Abdominal pain
Return of preoperative neurological symptoms
Prognosis
The prognosis for hydrocephalus depends on the cause, the extent of symptoms, and the timeliness of diagnosis and treatment. Some patients show a dramatic improvement with treatment while others do not. In some instances of NPH, dementia can be reversed by shunt placement. Other symptoms such as headaches may disappear almost immediately if the symptoms are related to elevated pressure. If the cause of NPH is known, the rate of shunting success can be as high as 80 percent. In cases in which the cause is unknown, the success rate varies from 25 to 74 percent.
In general, the earlier hydrocephalus is diagnosed, the better the chance for successful treatment. The longer the symptoms have been present, the less likely it is that treatment will be successful. Unfortunately, there is no way to accurately predict how successful surgery will be for each individual. Some patients will improve dramatically while others will reach a plateau or decline after a few months.
Shunt malfunction or failure may occur. The valve can become clogged or the pressure in the shunt may not match the needs of the patient, requiring additional surgery. In the event of an infection, antibiotic therapy may be needed. A shunt malfunction may be indicated by headaches, vision problems, irritability, fatigue, personality change, loss of coordination, difficulty in waking up or staying awake, a return of walking difficulties, mild dementia or incontinence. Fortunately, most complications can be dealt with successfully.
Hydrocephalus
What is the pituitary gland?
The pituitary is a small gland attached to the base of the brain (behind the nose) in an area called the pituitary fossa or sella turcica. The pituitary is often called the "master gland" because it controls the secretion of hormones. A normal pituitary gland weighs less than one gram, and is about the size and shape of a kidney bean.
The function of the pituitary can be compared to a household thermostat. The thermostat constantly measures the temperature in the house and sends signals to the heater to turn it on or off to maintain a steady, comfortable temperature. The pituitary gland constantly monitors body functions and sends signals to remote organs and glands to control their function and maintain the appropriate environment. The ideal "thermostat" setting depends on many factors such as level of activity, gender, body composition, etc.
The pituitary is responsible for controlling and coordinating the following:
Growth and development
The function of various body organs (i.e. kidneys, breasts and uterus)
The function of other glands (i.e. thyroid, gonads, and adrenal glands)
Pituitary Anatomy and Functions
The pituitary gland has two distinct parts: the anterior pituitary is closest to the front of the head, while the posterior pituitary is closest to the back of the head. These parts each contain unique cells and release different hormones that are responsible for specific control duties. The anterior pituitary is formed from the same tissue as the pharynx (the upper part of the mouth). The posterior pituitary develops from the bottom portion of the brain, and is actually an extension of the hypothalamus. The pituitary gland itself, is connected to and controlled by the hypothalamus, a region of the brain located right above the pituitary. The hypothalamus and pituitary together comprise the neuroendocrine system. The anterior pituitary accounts for about 80 percent of the pituitary gland, and is composed of the anterior lobe and the intermediate zone. The anterior lobe is responsible for the majority of the signaling hormones released into the blood stream. The posterior pituitary develops very early in life and does not produce any hormones of its own. It does contain the nerve endings of brain cells (neurons) that arise from the hypothalamus. These neurons produce the hormones vasopressin and oxytocin, which are transported down the pituitary stalk into the posterior pituitary. They are stored for later release into the bloodstream. The pituitary and hypothalamus work together to regulate the daily functions of the body, as well as play an essential role in growth, development and reproduction. The hypothalamus secretes hormones that control secretion of hormones from the anterior pituitary. The hypothalamic hormones are known as releasing hormones and inhibiting hormones, because of their influence on the anterior pituitary hormones. The pituitary gland performs its key functions by releasing several signaling hormones that consequently control the activities of other organs. The pituitary produces the following hormones: Adrenocorticotropic Hormone (ACTH) ACTH triggers the adrenals to release hormones such as cortisol and aldosterone. These hormones regulate carbohydrate/protein metabolism and water/sodium balance, respectively.
What is a pituitary tumour?
A pituitary tumour is an abnormal growth of pituitary cells. Pituitary tumours can either be nonfunctional (that is they do not secrete hormones) or produce specific hormones, such as prolactin (causing infertility, decreased libido, and osteoporosis), growth hormone (causing acromegaly), ACTH (causing Cushing's), TSH (causing hypothyroidism), or be nonfunctional (that is they do not produce hormones). These tumours behave according to their cell of origin and are named for the specific cell type affected. For example, if a tumour originates in a prolactin producing cell, the patient develops a prolactinoma-a prolactin secreting pituitary tumour that is common and usually treatable. High prolactin levels suppress production of the pituitary hormones (luteinizing hormone and follicle stimulating hormone) that stimulate production of estrogen or testosterone. Men with these tumours have low testosterone levels and lose their sex drive and eventually their masculine characteristics-hair, muscle, erections, and ability to produce sperm. Women with prolactin producing tumours often do not ovulate, experience low estrogen levels, and cease having menstrual periods. In both cases, patients with low sex hormones develop osteoporosis. It is important to remember that most pituitary tumours are benign and cancer is very rare. They have variable patterns of growth and affect different people in vastly different ways. Some are small and incidental, while others are small but cause hormone excess. Others may be rapidly growing mass lesions.
Pituitary Adenomas
Pituitary adenomas are the fourth most common intracranial tumour after gliomas, meningiomas and schwannomas. The large majority of pituitary adenomas are benign (not malignant) and are fairly slow growing. Even malignant pituitary tumours rarely spread to other parts of the body. Adenomas are by far the most common disease affecting the pituitary. They more commonly affect people in their 30s or 40s, although they are diagnosed in children as well. Most of these tumours can be successfully treated. Pituitary tumours can vary in size and behavior. Tumour that produce hormones are called functioning tumours, while those that do not produce hormones are called nonfunctioning tumours.
Symptoms
tumours smaller than 10 mm are called "microadenomas" and often secrete anterior pituitary hormones. These smaller, functional adenomas are usually detected earlier because the increased levels of hormones cause abnormal changes in the body. Approximately 50 percent of pituitary adenomas are diagnosed when they are smaller than 5 mm in size. Adenomas larger than 10 mm (the size of a dime) are called "macroadenomas," and usually do not secrete hormones. These tumours are often discovered as they produce symptoms by compressing nearby brain or cranial nerve structures.
The symptoms of a pituitary tumour generally result from endocrine dysfunction. For example, this dysfunction can cause overproduction of growth hormones, as in acromegaly (giantism), or underproduction of growth hormones, as in hypothyroidism. Hormonal imbalances can impact fertility, menstrual periods, heat and cold tolerance, as well as affect the skin and body in other ways.
Because of the pituitary gland’s strategic location within the skull, tumors of the pituitary can compress important brain structures as they enlarge. The most common circumstance involves compression of the optic nerves, leading to a gradual loss of vision. This vision loss usually begins with a deterioration of lateral peripheral vision on both sides.
The presence of three or more of the following symptoms may indicate a pituitary tumor:
Vision problems (blurred or double vision, drooping eyelid)
Headaches in the forehead area
Nausea or vomiting
Impaired sense of smell
Sexual dysfunction
Depression
Fatigue
Infertility
Growth problems
Osteoporosis
Unexplained weight gain
Unexplained weight loss
Easy bruising
Aching joints
Carpel tunnel syndrome
Disrupted menses
Early menopause
Muscle weakness
Galactorrhea (Spontaneous breast milk flow not associated with childbirth or the nursing of an infant)
Diagnosis
When a pituitary tumor is suspected, a physician will perform a physical examination, as well as vision testing to detect visual field deficits, such as loss of peripheral vision. Hormone testing of the blood and urine and imaging studies of the brain are used to confirm diagnosis. The most accurate diagnostic imaging test is magnetic resonance imaging (MRI), performed with and without a contrast agent.
Treatment
Early intervention provides the best chance for cure or control of the tumour and its side effects. There are three types of treatment used for pituitary tumours: surgical removal of the tumour, radiation therapy using high-dose x-rays/proton beams to kill tumour cells, and medication therapy to shrink or eradicate the tumour.
pituitary Tumour
What is Chiari Malformation?
The Chiari (kee-ar-ee) malformations are congenital abnormalities of the posterior fossa (base of brain where the spinal column joins the skull). This usually causes a protrusion of the cerebellum through the bottom of the skull (foramen magnum) into the spinal canal. This results in a poor circulation of cerebrospinal fluid from the brain to the spinal cord.
Professor Hans Chiari was a German pathologist who first described these abnormalities of the brain at the junction of the skull with the spine in 1890. Another doctor, Arnold, later added to the type II description, hence the name “Arnold-Chiari Malformation.” Of the four classifications of Chiari, only two types of the malformation have practical importance, commonly referred to as Chiari type I and Chiari type II.
Type I Chiari malformation
The Chiari I malformations are a result of the smallest herniation of an otherwise normal hindbrain. The tonsils would normally be round but often become elongated as they protrude down the spinal canal. Diagnosis can be difficult because not all patients will have the classical sign of deeply herniated tonsils. The Chiari II is usually found in children with spina bifida or myelomeningocele. Not only is part of the cerebellum unusually low and lying below the bottom of the skull, but the brain stem can be malformed in several ways. This malformation is part of a much greater malformation of the nervous system. This type of Chiari malformation is correctly referred to as “Arnold-Chiari” malformation.
What symptoms can it cause?
Many people with Chiari I malformation have no symptoms. However, any of the following symptoms may occur, alone or in combination. Some of the symptoms are related to the development of a syrinx (a fluid filled cavity in the spinal cord).
Severe head and neck pain
An occipital headache felt at the base of the skull that is made worse by coughing, sneezing, or straining
Loss of pain and temperature sensation of the upper torso and arms (as a result of a syrinx)
Loss of muscle strength in the hands and arms (as a result of a syrinx)
Drop attacks – collapsing to the ground due to muscle weakness
Spasticity
Dizziness
Balance problems
Double or blurred vision
Hypersensitivity to bright lights
Type II Chiari malformation
This malformation is characterised by downward displacement of the medulla, fourth ventricle, and cerebellum into the cervical spinal canal, as well as elongation of the pons and fourth ventricle. This type occurs almost exclusively in patients with myelomeningocele. Myelomeningocele is a congenital condition in which the spinal cord and column do not close properly during fetal development, resulting in an open spinal cord defect at birth. Other abnormalities associated with myelomeningocele include hydrocephalus, cardiovascular abnormalities, imperforate anus as well as other gastrointestinal abnormalities, and genitourinary abnormalities.
Symptoms
The symptoms associated with a Chiari II malformation can also be caused by problems related to myelomeningocele and hydrocephalus. These symptoms include:
Alteration in the pattern of breathing, including periods of apnea (brief periods of cessation of breathing)
Depressed gag reflex
Involuntary, rapid, downward eye movements
Loss of arm strength
Type III Chiari malformation
This malformation includes a form of dysraphism with a portion of the cerebellum and/or brainstem pushing out through a defect in the back of the head or neck. These malformations are very rare and are associated with a high early mortality rate, or severe neurological deficits in patients that survive. If treatment is undertaken, then early operative closure of the defect is necessitated. Hydrocephalus, which is commonly present, must also be treated through shunting.
Additional severe birth defects are often present, which may require extensive treatment. Infants with Chiari III malformation may have life-threatening complications.
Type IV Chiari malformation
This malformation is the most severe form and the rarest. The cerebellum fails to develop normally. There may be other associated malformations of the brain and brainstem. Most babies born with this malformation do not survive infancy.
How is it evaluated?
There are several tests that can help diagnose and determine the extent of Chiari malformation and syringomyelia.
Brainstem auditory evoked potential (BAER): An electrical test to examine the function of the hearing apparatus and brainstem connections. This is used to determine if the brainstem is working properly.
Computed tomography scan (CT or CAT scan): A diagnostic test that creates an image by computer reconstruction of x-rays; it is particularly good at defining the size of the cerebral ventricles and showing an obvious blockage. It is less effective for analysis of the posterior fossa or the spinal cord.
Magnetic resonance imaging (MRI): A diagnostic test that produces three-dimensional images of body structures using magnetic fields and computer technology. It can provide an accurate view of the brain, cerebellum and the spinal cord, is very good at defining the extent of malformations, and distinguishing progression. The MRI provides more information than the CT scan when analyzing the back of the brain and spinal cord, and is usually the preferred test.
Myleogram: An x-ray of the spinal canal following injection of a contrast material into the CSF space; can show pressure on the spinal cord or nerves due to malformations. This test is performed less frequently now.
Somatosensory evoked potentials (SSEP): An electrical test of the nerves involved in sensation, which gives some information about peripheral nerve, spinal cord, and brain function.
Treatment
Treatment of Chiari malformations and syringomyelia is very dependent on the exact type of malformation, as well as progression in anatomy changes or symptoms.
Chiari I malformations that are asymptomatic should be left alone. There is no indication for "prophylactic" surgery on these. If the malformation is defined as symptomatic, or is causing a syrinx, treatment is usually recommended.
Chiari II malformations are treated if the patient is symptomatic, and physicians have determined that there are no complications from hydrocephalus. In some patients, consideration of a tethered cord is also explored. In many infants who become symptomatic from a Chiari II malformation, the symptom onset and progression are severe and rapid, and this requires an urgent or emergency approach.
Chiari malformation
What is Trigeminal Neuralgia?
Trigeminal neuralgia or tic douloureux is sometimes described as the most excruciating pain known to humanity. The pain typically involves the lower face and jaw, although sometimes it affects the area around the nose and above the eye. This intense, stabbing, electric shock-like pain is caused by irritation of the trigeminal nerve, which sends branches to the forehead, cheek, and lower jaw. It is usually limited to one side of the face.
Although trigeminal neuralgia cannot always be cured, there are treatments available to alleviate the excruciating pain. Anticonvulsive medications are normally the first treatment choice. Surgery can be an effective option for those who become unresponsive to medications or for those who suffer serious side effects from the medications.
The Trigeminal Nerve
The trigeminal nerve is the fifth of 12 pairs of cranial nerves in the head. It is the nerve responsible for providing sensation to the face. One trigeminal nerve runs to the right side of the head and the other to the left. Each of these nerves has three distinct branches. ("Trigeminal" derives from the Latin word "tria," which means three, and "geminus," which means twin.) After the trigeminal nerve leaves your brain and travels inside your skull, it divides into three smaller branches, controlling sensations throughout your face:
The first branch controls sensation in your eye, upper eyelid and forehead.
The second branch controls sensation in your lower eyelid, cheek, nostril, upper lip and upper gum.
The third branch controls sensations in your jaw, lower lip, lower gum and some of the muscles you use for chewing.
Prevalence and Incidence
Advanced age is a major risk factor for trigeminal neuralgia. The disorder is more common in women than in men and rarely affects anyone younger than age 50. Hypertension and multiple sclerosis are also risk factors. Trigeminal neuralgia is relatively rare. An estimated 45,000 people in the United States and an estimated one million people worldwide, suffer from trigeminal neuralgia.
While inheritance has not been conclusively established, there is evidence that trigeminal neuralgia is tied to family history. According to the Trigeminal Neuralgia Association, 4.1 percent of patients with unilateral trigeminal neuralgia and 17 percent of patients with bilateral trigeminal neuralgia report a family history of the disorder.
Causes
The pain associated with trigeminal neuralgia represents an irritation of the nerve. The cause of the pain usually is due to contact between a normal artery or vein and the trigeminal nerve at the base of your brain. This places pressure on the nerve as it enters your brain and causes the nerve to misfire.
Other causes of trigeminal neuralgia include pressure of a tumour on the nerve or multiple sclerosis, which damages the myelin sheaths. Development of trigeminal neuralgia in a young adult suggests the possibility of multiple sclerosis.
What symptoms can it cause?
Most patients report that their pain begins spontaneously out of nowhere. Other patients say that their pain follows a car accident, a blow to the face, or dental surgery. Most physicians and dentists do not believe that dental work can cause trigeminal neuralgia. In these cases, it is more likely that the disorder was already developing, and the dental work caused the initial symptoms to be triggered coincidentally.
Pain is often first experienced along the upper or lower jaw and many patients assume they have a dental abscess. Some patients see their dentists and actually have a root canal performed, which inevitably brings no relief. When the pain persists, patients realize the problem is not dental-related.
The pain of trigeminal neuralgia is defined as either classic or atypical. With classic pain, there are definite periods of remission. The pain is intensely sharp, throbbing and shock-like, and usually triggered by touching an area of the skin, or by specific activities. Atypical pain is often present as a constant, burning sensation affecting a more diffuse area of the face. With atypical trigeminal neuralgia, there may not be a remission period, and symptoms are usually more difficult to treat.
Trigeminal neuralgia tends to run in cycles. Patients often suffer long stretches of frequent attacks followed by weeks, months or even years of little or no pain. The usual pattern, however, is for the attacks to intensify over time with shorter pain-free periods. Some patients suffer less than one attack a day, while others experience a dozen or more every hour. The pain typically begins with a sensation of electrical shocks that culminates in less than 20 seconds, with an excruciating stabbing pain. The pain often leaves patients with uncontrollable facial twitching, which is why the disorder is also known as tic douloureux.
The symptoms of several pain disorders are similar to those of trigeminal neuralgia. Temporal tendinitis involves cheek pain and tooth sensitivity as well as headaches and neck and shoulder pain. Ernest syndrome is an injury of the styomandubular ligament, which connects the base of the skull with the lower jaw, producing pain in areas of the face, head and neck. Occipital neuralgia involves pain in the front and back of the head that sometimes extends into the facial region.
Diagnosis
Magnetic resonance imaging (MRI) can detect if a tumour or multiple sclerosis is irritating the trigeminal nerve. However, unless a tumour or multiple sclerosis is the cause, imaging of the brain will seldom reveal the precise reason why the nerve is being irritated. The vessel abutting the nerve root is difficult to see even on a high quality MRI. Tests can help rule out other causes of facial disorders. Trigeminal neuralgia is usually diagnosed based on the description of the symptoms provided by the patient.
Treatment
Years ago trigeminal neuralgia was not well understood and treatment was nearly nonexistent. Today, there are several effective ways to alleviate the pain, including a variety of medications. There are drawbacks to these medications other than side effects. Some patients may need relatively high doses to alleviate the pain and the side effects can become more pronounced at higher doses. Anticonvulsant drugs may lose their efficacy over time. Some patients may need a higher dose to reduce the pain or may need a second anticonvulsant, which can lead to adverse drug reactions. Many of these drugs can have a toxic effect on some patients, particularly people with a history of bone marrow suppression and kidney and liver toxicity. These patients must have their blood monitored to ensure their safety.
Surgery
If medications have proven ineffective in treating trigeminal neuralgia, there are several surgical procedures which may help control the pain. Surgical treatment is divided into two categories: percutaneous (through the skin) and open. In general, percutaneous approaches are preferred in older or medically frail patients, in patients with multiple sclerosis, or in individuals who have failed to attain pain relief from the open approach. The open approach is recommended for younger and healthier patients. All of the procedures have varying success rates and some side effects, such as recurrence of pain and facial numbness.
Trigeminal neuralgia
What is Arteriovenous Malformation?
Arteriovenous malformations (AVMs) are defects of the circulatory system that are generally believed to arise during embryonic or fetal development or soon after birth. Although AVMs can develop in many different sites, those located in the brain or spinal cord can have especially widespread effects on the body. Most people with neurological AVMs experience few, if any, significant symptoms. The malformations tend to be discovered only incidentally, usually either at autopsy or during treatment for an unrelated disorder. But for about 12 percent of the affected population these abnormalities cause symptoms that vary greatly in severity.
Normally, arteries carry blood containing oxygen from the heart to the brain, and veins carry blood with less oxygen away from the brain and back to the heart. When an arteriovenous malformation (AVM) occurs, a tangle of blood vessels in the brain or on its surface bypasses normal brain tissue and directly diverts blood from the arteries to the veins.
What symptoms can it cause?
Seizures and headaches are the most generalized symptoms. AVMs also can cause a wide range of more specific neurological symptoms that vary from person to person, depending primarily upon the location of the AVM. Such symptoms may include muscle weakness or paralysis, loss of coordination, difficulties carrying out tasks that require planning, dizziness, visual disturbances, problems using or understanding language, abnormal sensations (such as numbness, tingling, or spontaneous pain), memory deficits, mental confusion, hallucinations, or dementia.
Is there any treatment?
Medication can often alleviate general symptoms such as headache, back pain, and seizures caused by AVMs and other vascular lesions. However, the definitive treatment for AVMs is either surgery or focused irradiation therapy. The decision to perform surgery on any individual with an AVM requires a careful consideration of possible benefits versus risks. Because so many variables are involved in treating AVMs, doctors must assess the danger posed to individual patients largely on a case-by-case basis.
How is an AVM evaluated?
Physicians now use an array of traditional and new imaging technologies to uncover the presence of AVMs. Angiography provides the most accurate pictures of blood vessel structure in AVMs. The technique requires injecting a special water-soluble dye, called a contrast agent, into an artery. The dye highlights the structure of blood vessels so that it can be recorded on conventional X-rays. Although angiography can record fine details of vascular lesions, the procedure is somewhat invasive and carries a slight risk of causing a stroke. Its safety, however, has recently been improved through the development of more precise techniques for delivering dye to the site of an AVM. Superselective angiography involves inserting a thin, flexible tube called a catheter into an artery; a physician guides the tip of the catheter to the site of the lesion and then releases a small amount of contrast agent directly into the lesion.
Two of the most frequently employed noninvasive imaging technologies used to detect AVMs are computed axial tomography (CT) and magnetic resonance imaging (MRI) scans. CT scans use X-rays to create a series of cross-sectional images of the head, brain, or spinal cord and are especially useful in revealing the presence of hemorrhage. MRI imaging, however, offers superior diagnostic information by using magnetic fields to detect subtle changes in neurological tissues. A recently developed application of MRI technology—magnetic resonance angiography (MRA)—can record the pattern and velocity of blood flow through vascular lesions as well as the flow of cerebrospinal fluid throughout the brain and spinal cord. CT, MRI, and MRA can provide three-dimensional representations of AVMs by taking images from multiple angles.
A detailed history-taking of the patient's symptoms and a physical examination are done first, followed by any of several tests, such as x-ray studies, Computerised Tomography (CT) scans, Magnetic Resonance Imaging (MRI), and angiograms. All findings are used to evaluate the patient's symptoms, determine the tumour's exact location, and provide the physician with a tentative diagnosis of the tumour type. During surgery, ultrasound imaging may be used to pinpoint the tumour's precise location and help the surgeon plan his approach for its removal. If an emergency craniotomy is required, an extensive workup may not be possible.
